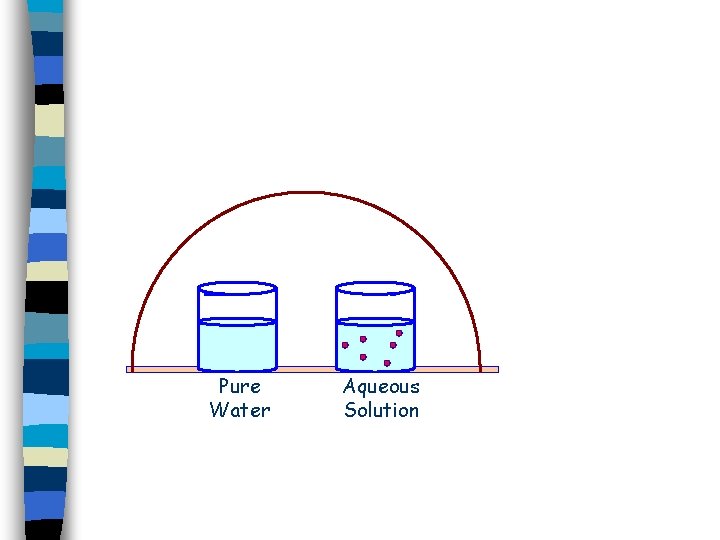
Therefore, pure water is homogeneous and pure substance However, when a homogeneous substance consists of two or more different types of molecules uniformly intermingled with one another, then it's called a homogeneous mixture A mixture's composition can vary, but a pure substance does not For instance, air is a homogeneous mixture ofGasoline homogeneous mixture, liquidliquid;Homogeneous Mixtures A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample It is often easy to confuse a homogeneous mixture with a pure substance because they are both

Matter Mixtures And Elements And Compounds You Will Distinguish Between Physical And Chemical Properties You Will Classify Matter By Composition Ppt Download
Is sugar and pure water a homogeneous mixture
Is sugar and pure water a homogeneous mixture-Sugar pure substance, compound;Grain alcohol pure substance, compound;



A Description Of Matter
Is water a mixture?Salt water mixture Is white sugar a homogeneous mixture? Sugar is a pure substance Because, it is composed of molecules of only one compound, i e, sugar (C12H22O11) So, it is not at all a mixture, it is a pure substance Every pure substance is homogeneous Because, composition and property of different portions of the substance are same through out the substance Hope, this helps
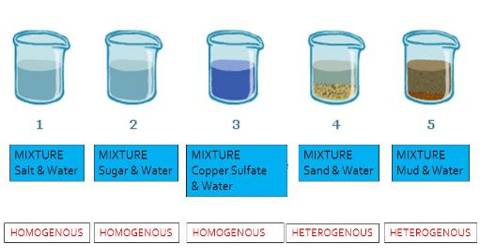
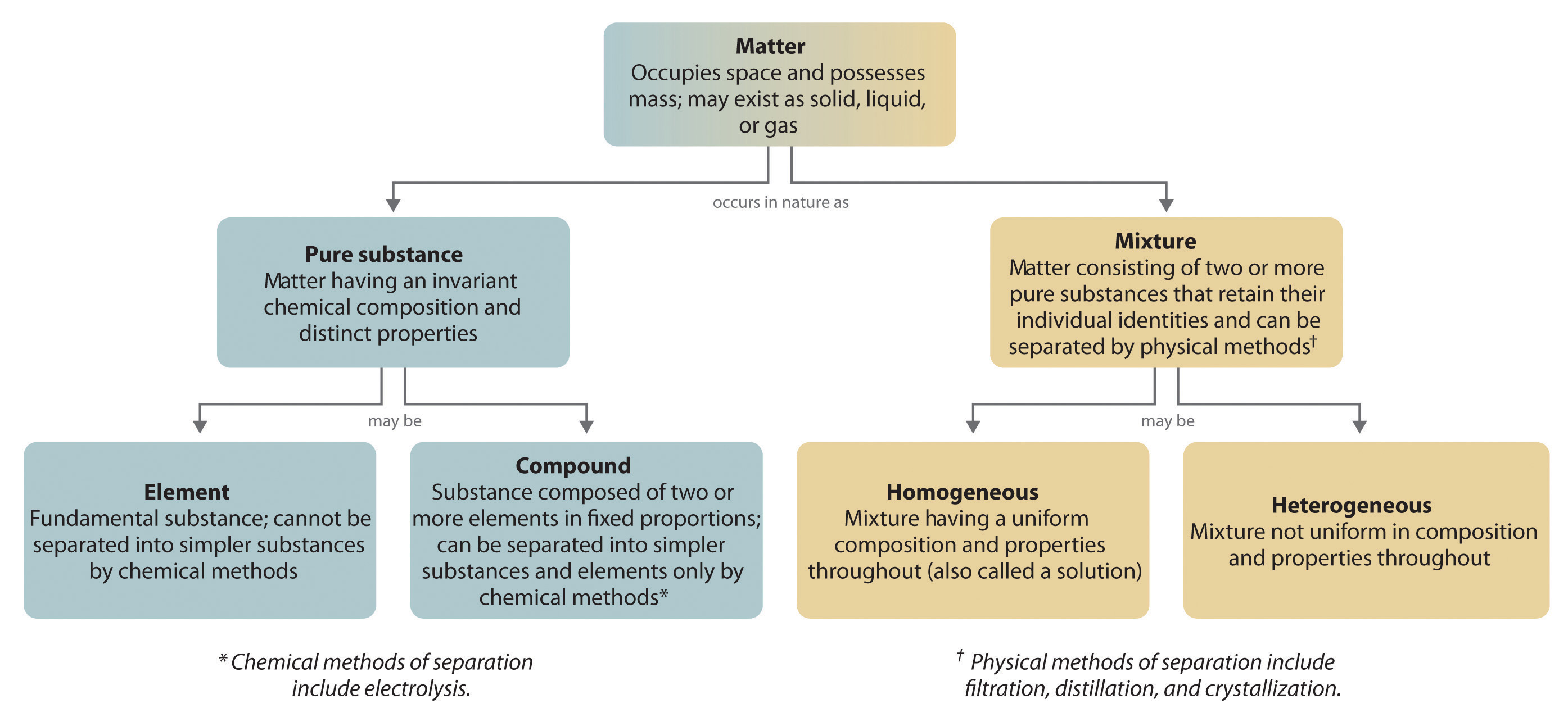
Characteristics of mixtures Mixtures can be characterized by being separable by mechanical means eg heat, filtration, gravitational sorting, centrifugation etc Mixtures can be either homogeneous or heterogeneous' a mixture in which constituents are distributed uniformly is called homogeneous, such as salt in water, otherwise it is called heterogeneous, such as sand in waterWater is a pure substance Salty water is a mixture Sodium chloride is a pure substance When dissolved in water the dissolved salt water is a mixture Pure substances comprise of only a single substance Eg Chlorine, Sodium, Sodium Chloride, Gold, Hydrogen, Helium, Mercury etc are pure substances when they are not mixed with anything elseA kind of mixture in which the different parts are evenly mixed and you can't pull separate particles out or even see separate particles is a chocolate chip cookie homogeneous or hetero or a pure substance?
Homogeneous Mixtures A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pureSugar is a pure substance Because, it is composed of molecules of only one compound, i e, sugar (C12H22O11) So, it is not at all a mixture, it is a pure substance Every pure substance is homogeneous Because, composition and property of different portions of the substance are same through out the substancePure substances contain atoms or molecules of only one kind Like distilled water has only water molecule and no impurities On the other hand, there are homogeneous mixtures



Homogeneous Mixture Experiment Qs Study




Chemistry The Study Of Matter Ppt Download
Salt water is made by mixing salt (NaCl) in water It is a homogeneous mixture and a heterogeneous one A difference between the two is For example, a mouthwash is a homogeneous mixture because it is a clear solution as all the components are evenly distributed On the other hand, a heterogeneous mixture is defined as the mixture in which solvent particles are unevenly distributed into the solvent Is ice cubes in liquid water a homogeneous or heterogeneous mixture?Water pure water is a compound, tap water is a mixture;




Classifying Matter Worksheet




Chapter 2 Is Matter Around Us Pure Class 9
All but the purest water contains dissolved minerals and gases; D pure liquids 8Water is a A homogeneous mixture B hetrogenous mixture C colloid D compound 9Colloidal particles can be easily seen with A a naked eye B an electron microscope C a microscope D a telescope Pure Substance or Mixture Element, Compound, Homogeneous, Heterogeneous;



How It Works Distillation And Filtration Mixtures Homogeneous Vs Heterogeneous Separating Mixtures




This Presentation Discusses Homogeneous And Heterogeneous Mixtures Provides Examples Explains How A Particle Di Heterogeneous Mixture Pure Products Mixtures
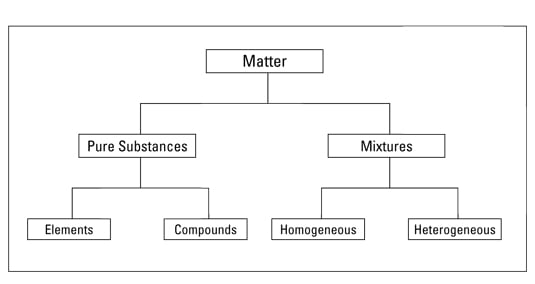
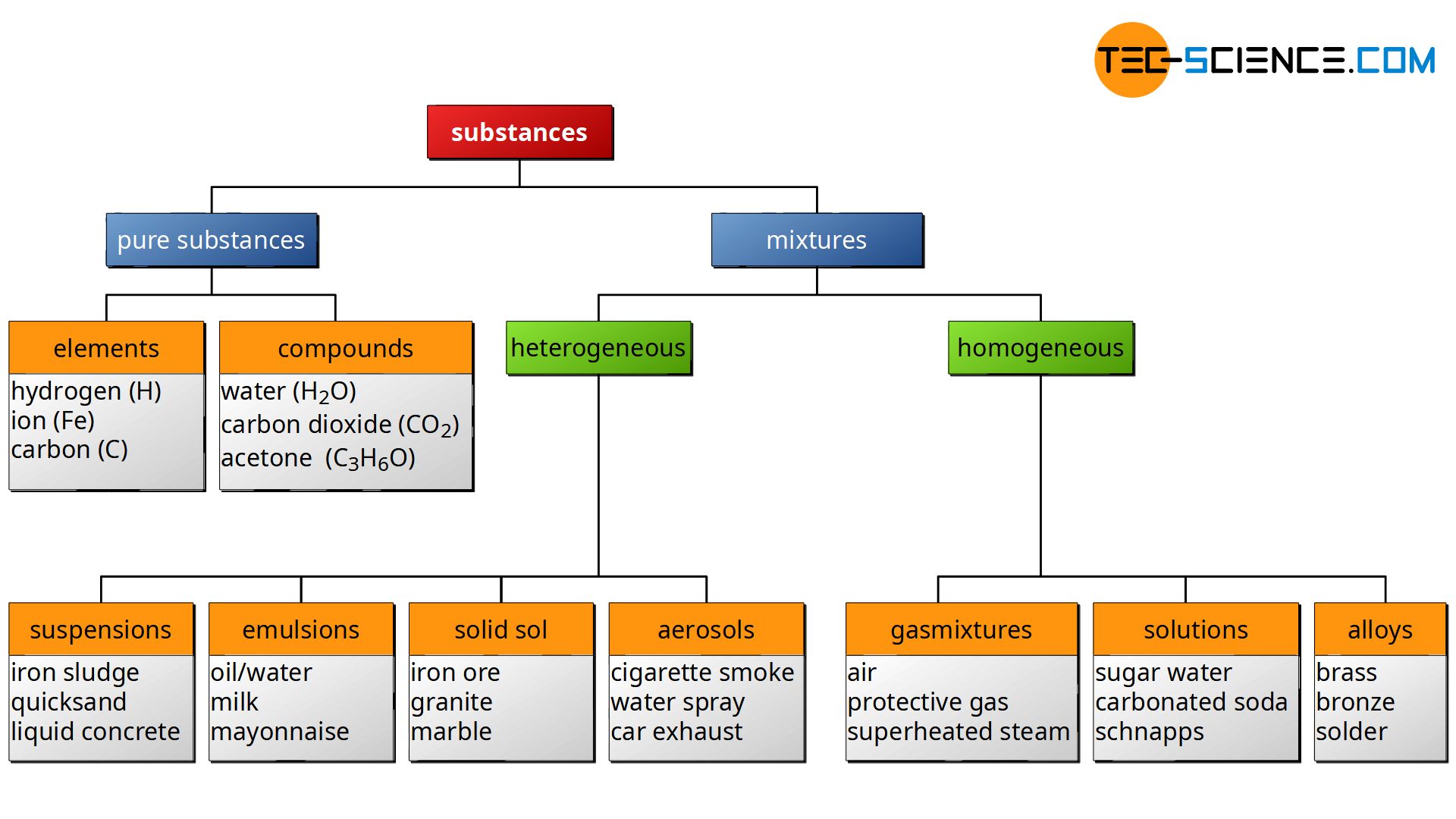
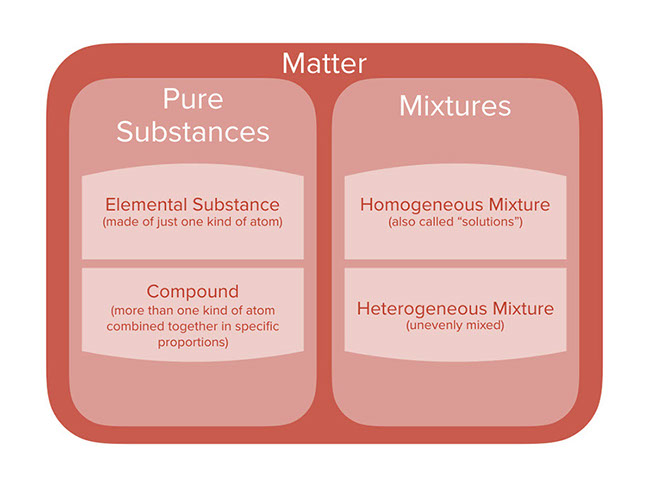
1A pure substance is a form of matter that has a fixed chemical composition and a distinct characteristic while a homogeneous mixture is a mixture of two or more compounds with compositions that are uniform or mixed together in such a way that they are indistinguishable from each other 2A pure substance cannot be separated into two or moreGold pure substance, element;Water, H 2 O, is a pure substance, a compound made of hydrogen and oxygen Although water is the most abundant substance on earth, it is rarely found naturally in its pure form Most of the time, pure water has to be created Pure water is called distilled water or deionized water




A Description Of Matter



Homogeneous Mixture Experiment Qs Study
Only when it is possible to separate the water from the salt is it confirmed that it was a homogeneous mixture and it was not pure water Characteristics of homogeneous mixtures They are made up of at least two components To be a mixture, a material must be made up of at least two substances, otherwise it would be a pure substance A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pure substance, because they are both uniformA homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform



1




Classification Of Matter Chemistrygod
Air in its pure form is a homogeneous mixture of gases like nitrogen, oxygen, carbon dioxide, argon and various other gases in smaller amounts However it becomes heterogeneous when it is polluted and additional harmful elements are added to it Ice in WaterWater another example of homogeneous mixture; Is a cookie a homogeneous mixture?



Http Arthurscience Weebly Com Uploads 5 0 9 2 Classifying Matter Ws Answers Pdf




Ws Classifying Matter Answers Mixture Solution
Mercury pure substance, element;Heterogeneous mixture sand in water pure substance compound salt homogeneous mixture Carbonated drink (a soda like Coke or Pepsi) pure substance element silver heterogeneous mixture The most abundant substance in a homogeneous mixture is called the solvent and the substance which dissolves in it is called a dissolved substance In the previously discussed example, water is the solvent, while sugar is the solute that was dissolved




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Review And Practice On Classification Of Matter
By definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is • Pure substance cannot be separated into two or more substances by any mechanical or physical method In contrast, substances in a homogeneous mixture can be separated by some methods • Pure substances have a fixed chemical composition compared to homogeneous mixturesPure Water is a pure substance It contains only water molecules The terms homogeneous and heterogeneous are not usually used to describe pure substances A mixture of water and salt is




Is Tap Water Homogeneous Or Heterogeneous Quora




Homogeneous Mixture Definition Examples Tutors Com
The homogeneous mixture is the combination of two or more pure substance in such a uniform manner that each of the substance is indistinguishable from the other substance The homogeneous mixture is only in the one phase of matter On the other hand, the pure substances in the heterogeneous mixture are not uniformly distributed, and it resultsMore specifically, because water is composed of hydrogen and oxygen, it is a compoundHomogeneous mixtures are those in which the components mixed are uniformly distributed throughout the mixture, they are uniform throughout Ice is a homogeneous mixture since particles are distributed uniformly within it In air, all gases would have a uniform composition Therefore, the air is an example of homogeneous mixture




Pure Substances Mixtures And Solutions Oh My Ppt Download



Http Www Duxbury K12 Ma Us Cms Lib2 Ma Centricity Domain 462 Chapter 15 elements and compounds Pdf
An example of a homogeneous compound is pure water (H 2O H 2 O) The hydrogen is bonded to the oxygen Carbon dioxide is another example of a homogeneous compound However, the air you are breathing is a homogeneous mixtureWhole milk is actually a heterogeneous mixture composed of globules of fat and protein dispersed in water Homogeneous mixuters are those in which the components are evenly distributed over the major component/constitute of the mixture A mixture is a material made of two or more types of molecules or substances that are not chemically combined The molecules or atoms making up a homogeneous mixture are distributed evenly, all in the same phase (Homogeneous mixtures can sometimes be mistaken for pure substances because of their uniform appearance) Salt water solution is a homogeneous mixture, for example, but salt mixed with sand is a heterogeneous mixture




Chemistry Classifying Matter




Homogenous And Heterogenous Mixtures Chemistry For Non Majors
No, water is not a mixture Water is a pure substance because it only contains one type of molecule, ie, H2O Another point to think about is that a mixture is produced when two substances are physically combined, not chemically What would happen if you release hydrogen and oxygen from its cylinder in the environment?A chocolate chip cookie is homogeneous because you can pull out the separate parts of the cookiePure substances are mostly homogeneous in nature containing only one type of atoms or molecules




Is Water A Pure Substance Or A Mixture Teach Besides Me




Homogeneous Mixture Definition Examples Tutors Com
These are dissolved throughout the water, so the mixture presents in the same phase and is homogeneous pls upvote me,Tap water in a glass Mixture Homogeneous soil Mixture Heterogeneous pure water (H2O) Pure Substance Compound, chromium (Cr) Pure Substance Element, Even the most pure water will contain dissolved gases from the air Impurities in a substance will affect its properties For example, they may change its boiling point What do all pure substances have in common?




Classification Of Matter Chemistrygod




Solution Solution Compound Element Pure Substance Chegg Com
Homogeneous Heterogeneous Homogeneous Mixture Mixtures having a uniform composition all through the substance are called Homogeneous Mixtures For instance – a mixture of salt and water, a mixture of sugar and water, air, lemonade, soft drink water, and so on Here, a classic example is the mixture of salt in water A solution of salt dissolved in water is an example of a homogeneous mixture When the salt dissolves, it spreads evenly through the water so that all parts of the solution are the same, and you can no longer see the salt as being separate from the waterA solution is a homogeneous MIXTURE of two or more substancesWater is a compound, a pure substance It is made of then elements hydrogen and oxygen, but shares none of their properties Pure water is a solvent, not a solution




Solved Classify Each Of The Following As A Pure Substance Or A Mixture If A Mixture Indicate Whether It Is Homogeneou



How To Distinguish Pure Substances And Mixtures Dummies
Saltwater is a homogeneous mixture, or a solution Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture Water is a substance;A Solution is a mixture of two or more substances Solute The substance that is being dissolved – the smallest amount of the two Solvent The substance that is doing the dissolving – the larger amount of the two Water is our universal solvent Question KoolAid and water – Which is the solvent?Vinegar is an example of a homogeneous mixture , and not a pure substance since water, its solute, is dissolved in the solvent, being acetic acid Homogeneous mixtures are also known as solutions, which are mostly composed of liquids (including vinegar ), but can include gases



1
-and-Milk-(left).jpg?revision=1)



1 3 Classification Of Matter Chemistry Libretexts
A mixture in which its constituents are not distributed uniformly is called heterogeneous mixture, such as sand in water One example of a mixture is air Air is a homogeneous mixture of the gaseous substances nitrogen, oxygen, and smaller amounts of other substances Mixtures can have any amounts of ingredientsHomogeneous mixtures are mixtures in which the constituents don't appear separately blood a sugar solution when the sugar is completely dissolved a mixture of alcohol and water a glass of orange juice salty water (where the salt is completely dissolved) brewed tea or coffee soapy waterWhich is the solute?




The Homogeneous And Heterogeneous Mixture Diagram Quizlet




Classification Of Matter Chemistrygod
At that point, I wasn't sure if the concrete was a homogeneous or heterogeneous mixture Concrete is a heterogeneous mixture It is composed of cement, water, and aggregate that can be crushed stone, sand, and/or gravel Concrete is made up of different compounds such as solids – aggregate – and liquid – waterBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase




Matter Mixtures And Elements And Compounds You Will Distinguish Between Physical And Chemical Properties You Will Classify Matter By Composition Ppt Download



Classification Of Matter Tec Science




Homogeneous Mixture Definition Examples Tutors Com




8 Classify Each Of The Following As A A Chegg Com




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




Is Pure Water Perfectly Homogeneous Quora



Difference Between Pure Substance And Mixture Definition Composition Properties Examples




Is Sand And Water A Homogeneous Mixture Quora




Case Of Pure Substances Answer The Following Questions In Detail 1 Classify The Following As Pure Substances Or Mixtures In Case Of Pure Suhet Whether They Are Element Or Compound But In



3




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends




Lesson Explainer Mixtures Nagwa
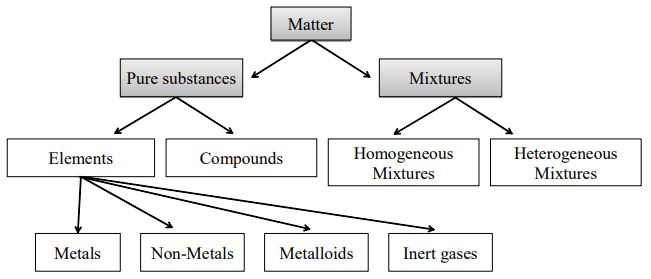



Is Matter Around Us Pure Practically Study Material




Question 4 Give The Classification Of Matter When Two Substances Exist With Two Phases Present A Homeworklib



Homogeneous Heterogeneous Mixture Definition Examples Selftution
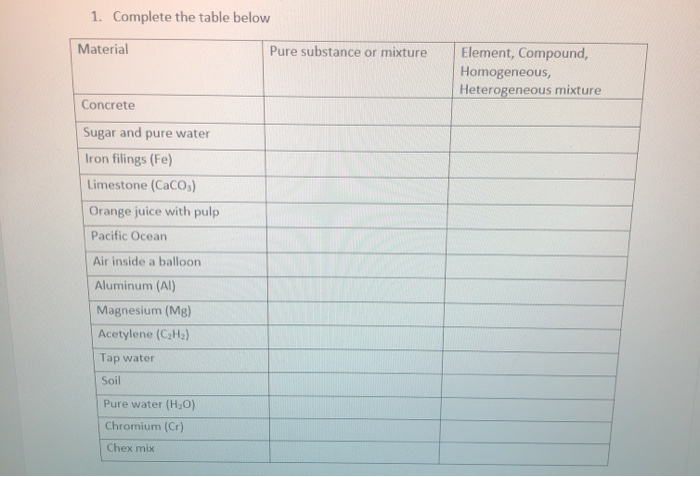



1 Complete The Table Below Material Pure Substance Chegg Com
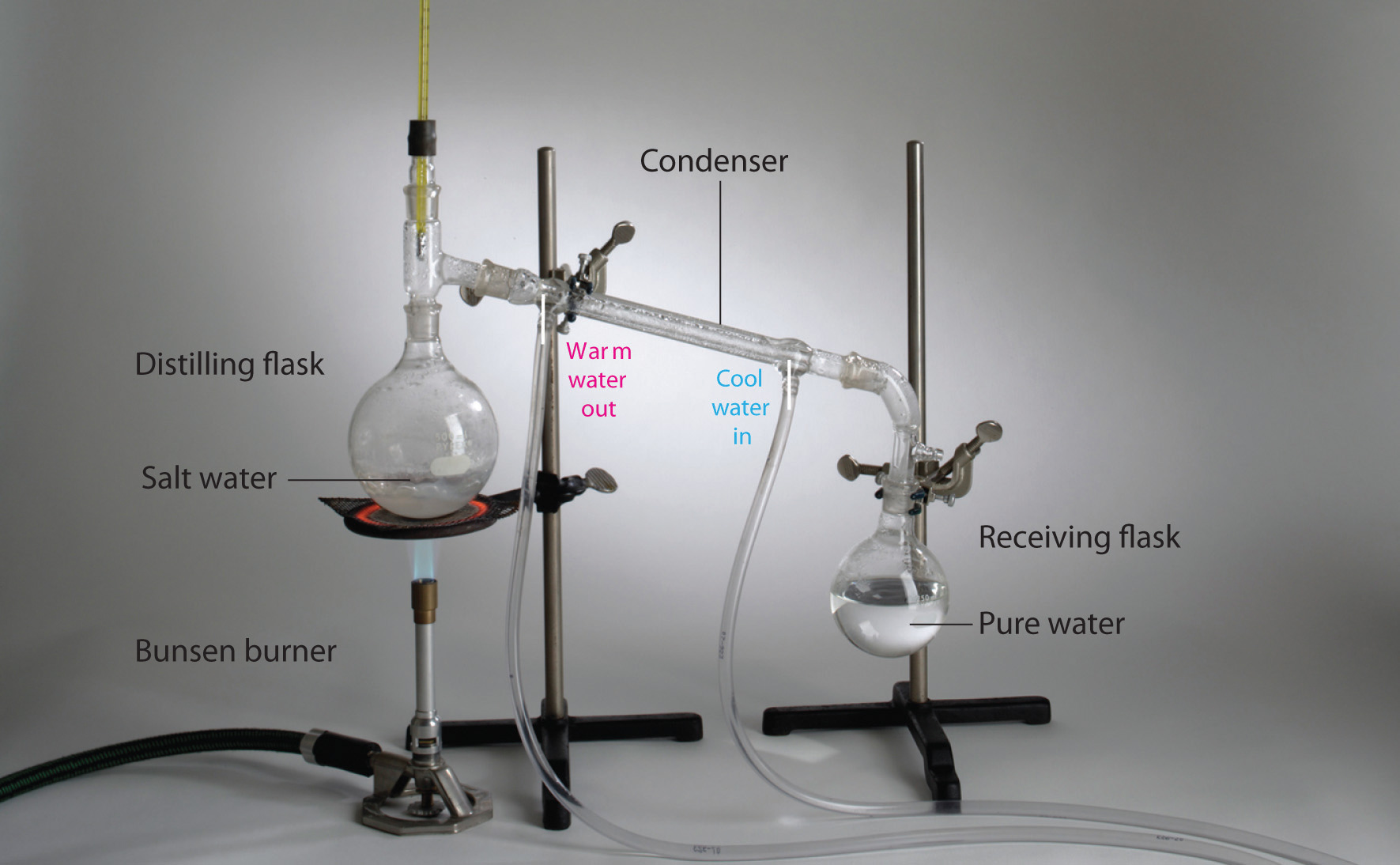



1 2 Classification Of Matter Chemistry Libretexts
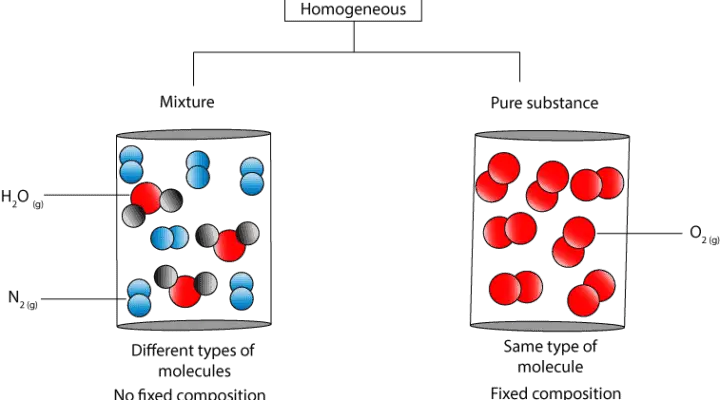



If A Substance Is Homogeneous Is It A Pure Substance




Chapter 1 Section 2




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends



Solutions N Solution A Homogeneous Mixture Of Pure



3 5 Separation Of Mixtures Chemistrysaanguyen



Mixture




Compounds Elements And Mixtures Ppt Download



Solutions



Mixture




Examples Of Pure Substances




What Is The Difference Between Pure Substances And Mixtures




Table 1 Identification Of Matter Item Pure Substance Chegg Com
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




Please Answer The Below Given Questions Fast I Need These Within 15 Minutes Pleaseee Brainly In




How Are Mixtures And Pure Substances Alike



1 3 Classification Of Matter Chemistry Libretexts




What Is A Homogeneous Mixture Definition And Examples




3 Pure Substances And Mixtures Pdf Free Download




Classifying Matter Mixture Solution
/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)



What Are Examples Of Pure Substances




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Types Of Mixtures Science At Your Doorstep




Chapter 1 Section 2




Pure Substances And Mixtures Unit 2 Solution Solvent




Pure Substance Heterogeneous Mixture Or Homogeneous Mixture Flashcards Quizlet



Is Sugar A Homogeneous Or Heterogeneous Mixture Quora



Lesson Categories Of Chemicals And Mixtures
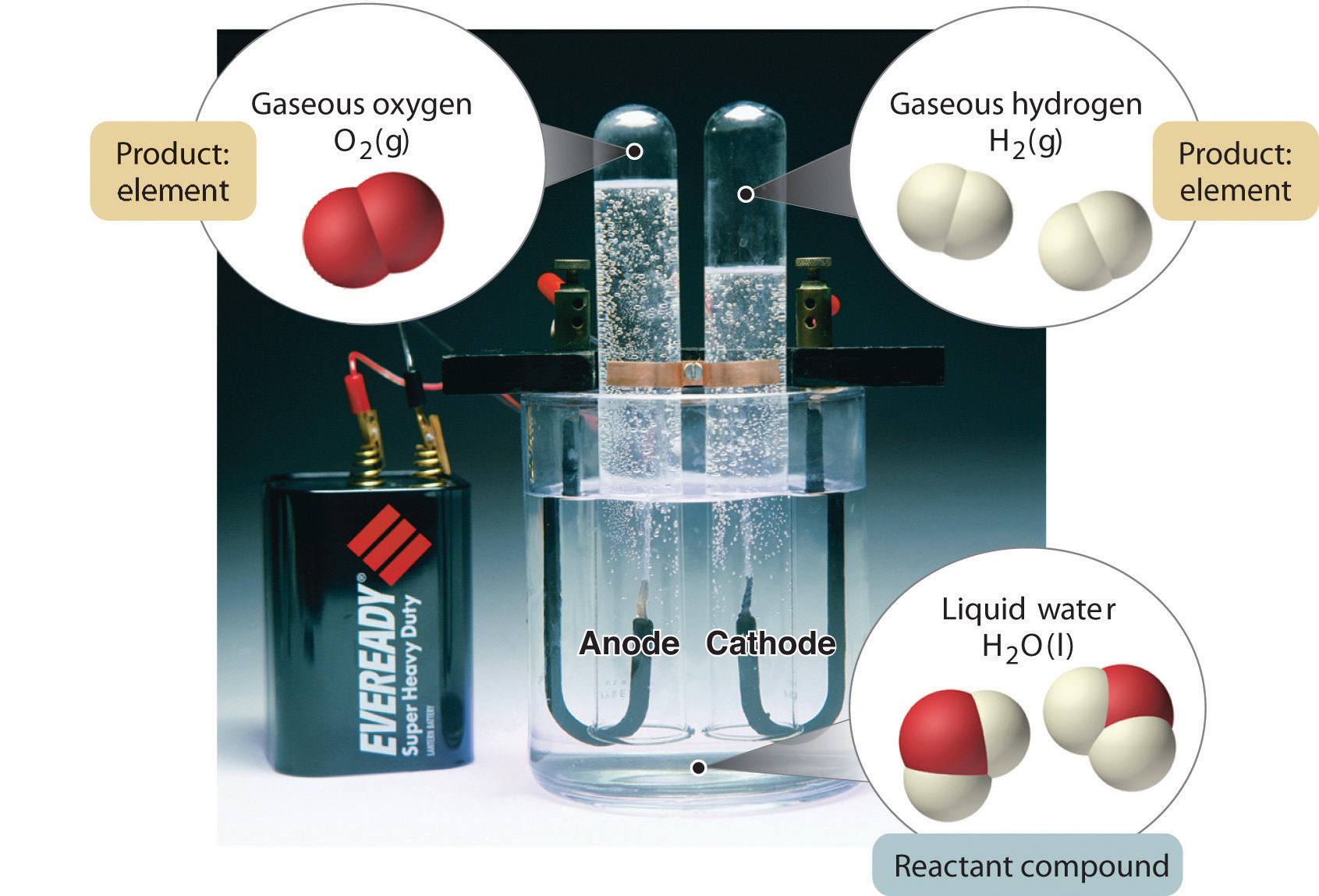



A Description Of Matter




5 Pure Water Can Be Described As Check All That Apply Mixture Pure Brainly Com




Pure Substances And Mixtures Unit 2 Matter Element



File




Matter Mixtures Pure Substances Elements Compounds Heterogenous Ppt Video Online Download



Pure Substances Mixtures Multiple Choice Identify The Choice That



Is Ethanol And Water A Homogeneous Mixture Quora




Compounds Mr Wilson World Famous Teacher




Ws Classifying Matter Answers




Pure Substances And Mixtures




Substances Vs Mixtures Mixture Homogeneity And Heterogeneity




Iii Write True Or False 1 Mixture Is A Pure Substance Lf2 Alloys Are Homogeneous Mixtures Of Two Brainly In
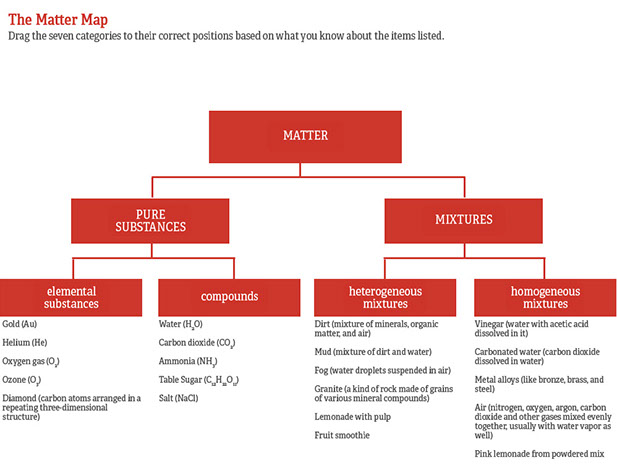



Lesson Categories Of Chemicals And Mixtures




Examples Of A Heterogeneous Mixture Of A Pure Substance




3 4 Classifying Matter According To Its Composition Chemistry Libretexts



Pure Substances And Mixtures Neds Declassified




3 5 Pure Substances And Mixtures Chemistry Libretexts




Classifying Matter 1 Classify Each Mixture As Chegg Com




Which Of The Following Best Describes This Substance A A Clutch Prep




Elements Compounds And Mixtures Ppt Video Online Download



Schoolwires Henry K12 Ga Us Cms Lib Ga Centricity Domain 9970 Classifying matter Pdf
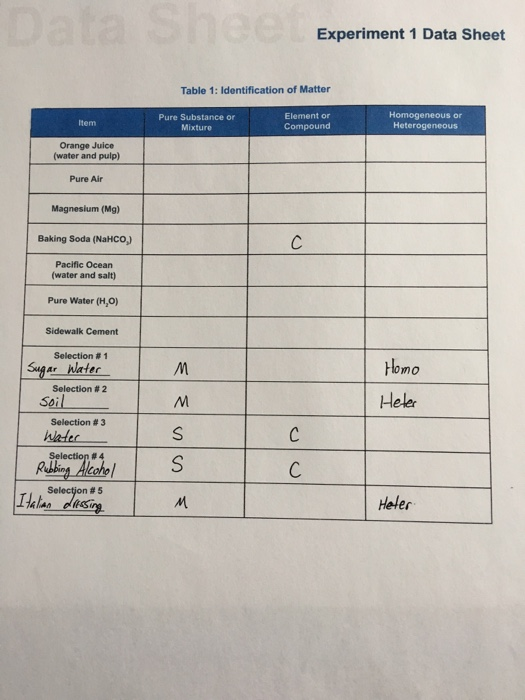



Experiment 1 Data Sheet Table 1 Ldentification Of Chegg Com




Chemistry For Kids Chemical Mixtures




Chemistry Classifying Matter Classify Each Of The Chegg Com




Pure Substances And Mixtures Unit 2 Solution Solvent



1



Is Sugar A Homogeneous Or Heterogeneous Mixture Quora



Mixture



0 件のコメント:
コメントを投稿